Product
Lead Liposomal Drug Product - LC101
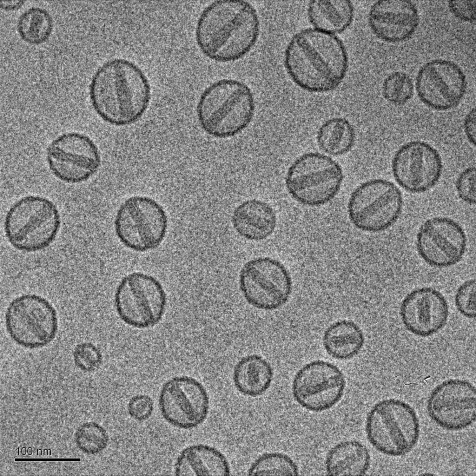
Drug Product: Sterile Doxorubicin-HCl Liposome injection
Indications: Ovarian, Multiple Myeloma and AIDS related sarcoma
Mechanism of Action:
The porous blood vessels at the tumor tissue supports relocalization of the Doxorubicin-loaded nano-liposomes at the tumor site
Ammonia unique to tumor metabolome induces selective Doxorubicin release of the liposomes at the tumor siteProviding therapeutic efficacy with reduced exposure of normal healthy tissues and organs (lower Cardiotoxicity – the major toxic effect of Doxorubicin)
Commercial production: CMO providing cGMP manufacturing and release of LC101, meets regulatory requirements (last FDA audit 2019)
The Production process
Therapeutic Merit
Index:
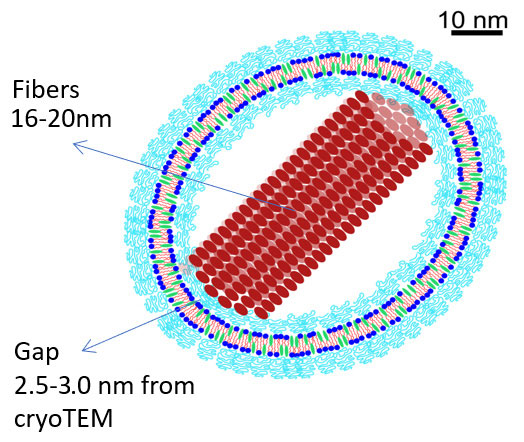
- Blue – The head-groups of HSPC and the phosphoethanolamine moiety of the DSPE-PEG
- Red – Acyl tails
- Cyan – PEG chains
- Turquoise – Cholesterol molecules
- Bourdeaux – The unit cells of doxorubicin
* The illustration is drawn to scale as indicated by the scale bar and was created by Einav Raviv. (Schilt et al BBA 2015)